The use of lithium metal foil as a battery anode shows great promise for increased energy capacity and power output. The problem is that during charging, the lithium metal ions that travel from the cathode through the electrolyte do not evenly attach themselves to the lithium metal that makes up the anode. Instead, they form spiky crystalline dendrite structures. These dendrites can grow large enough to reach the cathode and short out the battery, sometimes causing a fire.
One way to prevent dendritic growth on the surface of lithium metal anodes is the use of a solid electrolyte. Solid electrolytes made from polymer or ceramic materials have been shown to help reduce the growth of dendrites. There are problems, however. The anode and cathode of the lithium battery change volume when they receive or give up lithium ions during charging and discharging. This change makes a ceramic solid electrolyte prone to cracking, causing a degradation of performance. Polymer electrolytes, however, can have the flexibility necessary to adapt to the changes in electrode volume during the battery operation.
The downside to a polymer electrolyte is its poor ionic conductivity and weak mechanical properties. A great amount of research is underway to examine the incorporation of inorganic fillers to help improve the ionic transport and mechanical strength of polymer electrolytes.
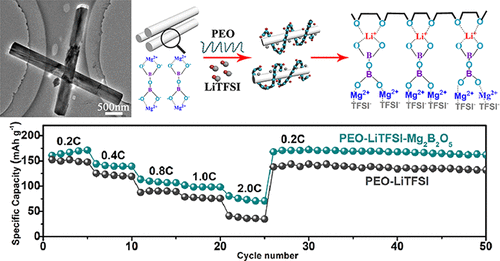
Now, as reported in Nano Letters of the American Chemical Society (ACS), a group of researchers from Zhejiang University of Technology in China has incorporated nanowires made from magnesium borate (Mg2B2O5) into a polyethylene oxide (PEO) based solid-state electrolyte. The wires were approximately 200 nm in diameter and 4,000 nm long.
The researchers examined loadings of 5%, 10%, 15%, and 20% of nanowires into the solid polymer electrolyte. The addition of the nanowires to the polymer electrode were found to increase the number of lithium ions and allow them to move through the material at a faster rate. The mechanical properties of the polymer electrolyte were also enhanced by the addition of the nano-materials, as was its flame resistance when exposed to fire.
The researchers attributed the increase in ionic conductivity to the improved motion of the PEO polymer chains and the increase in the number of lithium ion pathways afforded by the nanowires. An experimental battery using a lithium metal anode and LiFePO4 cathode demonstrated both improved rate performance and higher cyclic capacity when compared to a battery without the nanowires.
Although the research reported is quite preliminary, the concept of incorporating nano-materials into solid-state polymer electrolytes may provide another avenue to improve the safety and viability of lithium metal batteries.
