In March of this year, funding for the Joint Center for Energy Storage Research (JCESR) was approved by Congress for another year. The $24.1 budget for 2018 from the US Department of Energy (DOE) allows JCESR to continue its work on “beyond lithium” batteries. The DOE was also directed to move forward with a five-year renewal of JCESR. The review of that proposal is currently underway.
JCESR got its start in 2012, when it was funded for five years with the vision, “to create game-changing, next-generation battery technologies that will transform transportation and the electricity grid the way lithium-ion batteries transformed personal electronics.” The center is administered out of Argonne National Laboratory in Illinois, but includes collaborations with researchers, scientists, and engineers at national laboratories and universities around the country, as well as a few private startup companies.
From the beginning, the JCESR had ambitious goals. For the transportation sector, the objective was to create batteries with five times the energy density at one fifth the cost. This would allow an electric vehicle (EV) to easily and inexpensively travel up to 400 miles on a single charge. For electrical power grids, the same energy density and cost goals would make storing and releasing renewable electricity on the grid as inexpensive as generating it with a natural gas turbine.
Pursuing fundamental research, JCESR focused on three concepts to redefine battery energy storage:
- Multivalent Intercalation: Lithium ions are limited to a single charge state and can only gain or lose one electron. Other elements can gain or lose two or three electrons, increasing the number of electrons available from the same size battery. JCESR conducted a computer screening of more than 1,800 multivalent compounds, identifying calcium and magnesium (each with two electrons to transfer) as the most promising new battery materials.
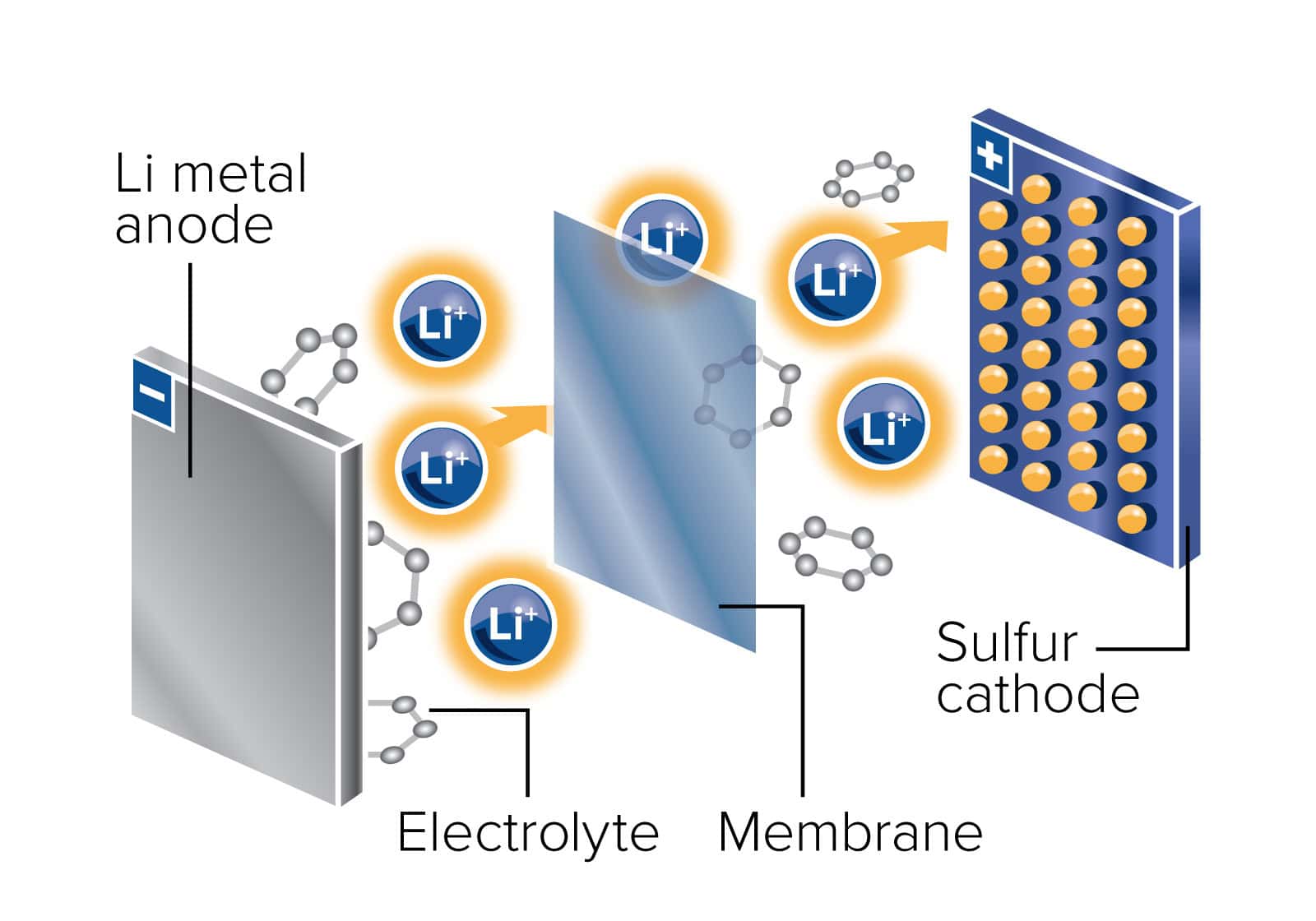
- Chemical Transformation: Current lithium ion batteries insert lithium ions between layers of a carbon graphite anode during charging in a process called intercalation. If the graphite anode can be replaced by lithium metal, a true chemical reaction can occur at the anode, producing higher energy densities. JCESR is focused on a lithium metal anode and a sulfur cathode as a possible future battery system.
- Redox Flow-Flow Batteries: These batteries use energy dense liquids that undergo reduction and oxidation (redox), allowing them to store electrical energy during charging and release it during discharging. The liquids are held in storage tanks and are pumped through a reaction cell. Flow batteries have a great deal of potential for grid storage. JCESR is using inexpensive and versatile organic molecules as the energy storing redox materials.
To investigate these three concepts, JCESR scientists use sophisticated computer simulation tools as well as multimodal characterization by NMR, electron microscopy, X-ray scattering, and scanning probes. JCESR “unifies discovery science, battery design, research prototyping, and manufacturing collaboration in a single highly interactive organization.”
By all accounts, JCSER has been successful. “We promised one prototype for the grid, and one for the car, at the beginning,” George Crabtree, Argonne National Laboratory senior scientist and distinguished fellow, and the director of JCSER, told Design News. “What we delivered was two for the grid and two for the car. So we delivered four, after we promised two. We made about 60% of the energy density goal. In other words, we got three times the energy density, not five. We made the cost goal within 20%—we wanted $100 per kilowatt-hour, we got $120,” Crabtree noted.
