Electrospinning uses electric fields to manipulate nanoscale and microscale fibers. The technique is well-developed but time-intensive and costly. A team from Michigan Technological University came up with a new way to create customizable nanofibers for growing cell cultures that cuts out time spent removing toxic solvents and chemicals. Their work is published in Materialia.
Smitha Rao, assistant professor of biomedical engineering at Michigan Tech, led the research. She said the approach is innovative, “we’re coming at this completely sideways,” and the team focused on streamlining electrospun nanofiber production. Nanofibers are used as scaffolds, made up of strands and pockets, that can grow cells.
“We want an assembled, highly aligned scaffold that has ideal structures and patterns on it that cells will like,” Rao said. “Take a cell, put it on porous materials versus elastic materials versus hard materials, and it turns out the cell does different things. Usually you use varied materials to get these diverse characteristics. Cells respond differently when you put them on different surfaces, so can we make scaffolds that provide these different conditions while keeping the materials same?”
In a nutshell, yes. And making customizable scaffolds is surprisingly simple, especially when compared to the laborious casting and additive processes typically used to produce scaffolds suitable for electrospinning. Plus, Rao’s team discovered a pleasant side effect.
“We take the polymers, then we put them into solutions, and we came up with this magical formula that works—and then we had to go electrospin it,” Rao explained, adding that the team noticed something odd during the process.
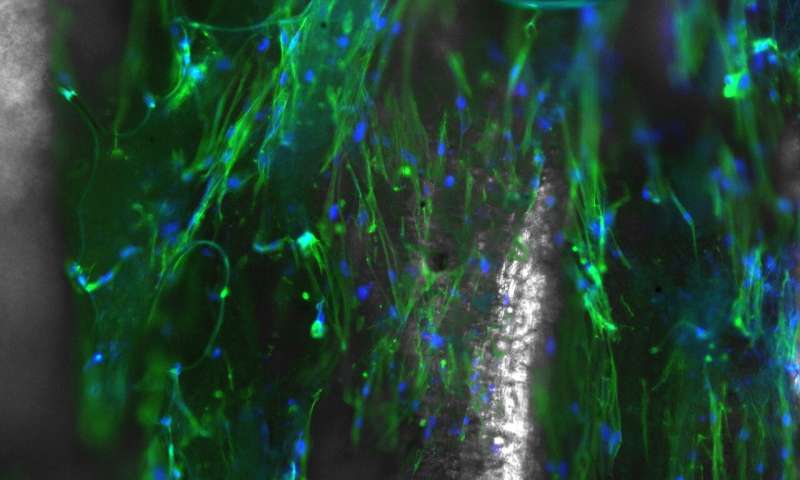
“We saw that the cells aligned without us applying anything externally. Typically, to make them align you have to put them in an electric field, or put them in a chamber and agitate the scaffold to force them to align in a particular direction by applying external stresses,” she said. “We’re basically taking pieces of this scaffold, throwing it in a culture plate and dropping cells on it.”
When spun in an electric field—imagine a cotton candy machine—the self-aligning cells follow the strand-and-pocket pattern of the underlying nanofibers. Rao’s team, including lead author and Ph.D. student Samerender Nagam Hanumantharao and masters student Carolynn Que, found that varying electric field strengths result in different pocket sizes. At 18 kilovolts, the magic happens and the fibers align just so. At 19 kilovolts, small pockets form, ideal for cardiac myoblasts. At 20 kilovolts, honeycombs of pockets expand in the fibers. Bone cells prefer the pockets formed at 21 kilovolts; dermal cells aren’t picky, but especially like the spacious rooms that grow at 22 kilovolts.
