Modern electric vehicle batteries began with lithium-ion batteries, originally applied to portable electronics and other portable applications. Batteries based on sodium have now been developed. These batteries have something in common. They all use intercalation. Intercalation simply means that charged compounds are physically stuffed in between the gaps in materials. One dictionary definition of intercalation is the insertion of a day into a calendar, and while I have often longed for an extra day in the week, this is not what it means for lithium batteries. It means the compound is inserted into the structure of another substance. This is fundamentally different from a lead-acid battery, which literally plates lead onto a metal plate. That process works like electroplating.
The type of battery used for EVs is not like that. Instead, it stuffs molecules into structures. Chemical transformations happen in the electrolyte.
A separator is used to keep the anode and cathode physically separate, and isolate the cathode from the anode. The separator is porous and allows the electrolyte to travel freely through it, along with the chemical compounds in it. The cathode is a metal oxide, and the anode is normally made of graphite, a form of carbon. Charge-carrying compounds can exist in the cathode and anode, and the amounts and locations are a function of the state of charge of the cell. Charging and discharging the cell drives those charged compounds between anode and cathode, allowing current to flow outside the cell and causing a voltage on the terminals. In construction of lithium cells, cathode metal oxides are deposited on a thin foil conductor, and graphite is deposited on the anode. The metal foil provides connection to terminals outside the cell.
All batteries are chemical devices that are governed by the Arrhenius equation. It is an equation that governs the rate of chemical reactions.
Chemical reactions are proportional to temperature by an exponent. In order to evaluate cells, accelerated lifetime testing is used. There are two variables in particular that accelerate cell aging, voltage and temperature.
This is why cell and pack temperatures, and charging, are carefully controlled.
The exponent relationship of temperature is convenient. It allows small changes in temperature to cause large changes in reaction rate. That provides a means for accelerated lifetime testing. This is one reason cell temperatures are carefully controlled in normal operation. At the other end of the spectrum, if cell temperatures are too low, reactivity is low, and battery operation is reduced. Both variables can be used to accelerate processes within the cell that lead to degradation. One of the factors that leads to capacity degradation is a reduction in available lithium to act as charge storage. Side reactions can bind to lithium, making it unavailable. Another factor in cell aging is related to other side reactions. There are compounds within the cell such as binders, electrolyte, and so forth, that are not intended to be involved in the chemical reaction. They can get broken down and become lodged in the cathode or anode, and internal resistance may increase. Electrolytes slowly degrade and block conduction. One of the pioneers of lithium batteries, Jeff Dahn, published papers which describe these matters. He pioneered a method of determining real-life battery cycle life by measuring coulombic efficiency, and using accelerated life testing. Coulombic efficiency is a way of determining how much the initial lithium available for charge transfer decreases.
Cells do not just die completely. Buildup of unwanted materials blocks conduction. They lose capacity and their internal resistance goes up. When the internal resistance is too high, operation becomes inefficient and it is difficult for the cells to deliver power. While the exact definition of cell life may be arbitrary, their performance is measurable.
In addition, if voltages and temperatures are too high, unwanted reactions may happen within the cell, releasing oxygen and combining with the contents, creating a runaway fire condition known as thermal runaway. At the other extreme, severe discharge may result in unwanted reactions within the cell, among them plating, which are irreversible, rendering the cell non functional.
These facts instruct how and why lithium battery charging works the way it does. They describe why thermal management is essential for cell life and optimum performance. They also describe why maximum charge, which is at maximum voltage, is avoided for long periods of time. Cell wear out is proportional to the amount of time under given conditions. That is why brief periods of full charge, with discharge following quickly afterwords, is recommended. For normal operation, charge to lower voltages produces much less wear-out, because wear-out is an exponential function of temperature and voltage. It also explains why cells not in use are stored at a mid charge of about 40%. It is a balance between avoiding full discharge due to self-discharge and passive wear-out due to voltage and time. Much of the confusion in lithium battery usage can be cleared by understanding of fundamental operation and governing equations.
Battery lifetime is measured in cycles, and is often referred to as capacity degradation, with a somewhat arbitrary limit of 80% of initial capacity per charge/discharge cycle in kWh. Warranties sometimes use 70% as a limit. What we mean by a cycle is a charge/discharge, which is one cycle.
Simply put, cell cycle life is the same, given the same cells and operating conditions. Certain details of cell design may trade one characteristic for another, but for a given formulation, the cell lifetime cycles are fixed.
For electric vehicles, the amount of vehicle miles traveled per cycle is proportional to the amount of battery capacity used. Under the same conditions, this result is broadly independent of temperature and other variables. This allows direct comparative analysis.
Battery Capacity (kWh) x vehicle constant (miles/kWh) = Vehicle Miles Traveled (miles), or Range
Since we know lifetime cycles, and we also know lifetime miles, and we know lifetime miles is fixed, for the same conditions of operation:
Lifetime miles = Range/cycle x Cycle life
Lifetime miles per pack is proportional to pack capacity.
These simple fundamental formulas have far reaching, yet often overlooked, consequences.
Power vs. Energy and Cost Trade-Off
High-charge-rate batteries are high-power batteries with low internal resistance. Let us examine the relationships.
“Lithium-ion batteries exhibit a well-known trade-off between energy and power, often expressed as the power-over-energy or P/E ratio, and typically represented in a so-called Ragone plot of power as a function of energy.”
“High-energy cells have typically thicker electrodes with a higher volume fraction of active materials, while HP cells have typically thinner electrodes with a higher volume fraction of electrolyte and conducting additive.”
In simple words, that means that low energy density cells exhibit lower Power over Energy ratios (P/E).
A plot of specific power vs specific energy:
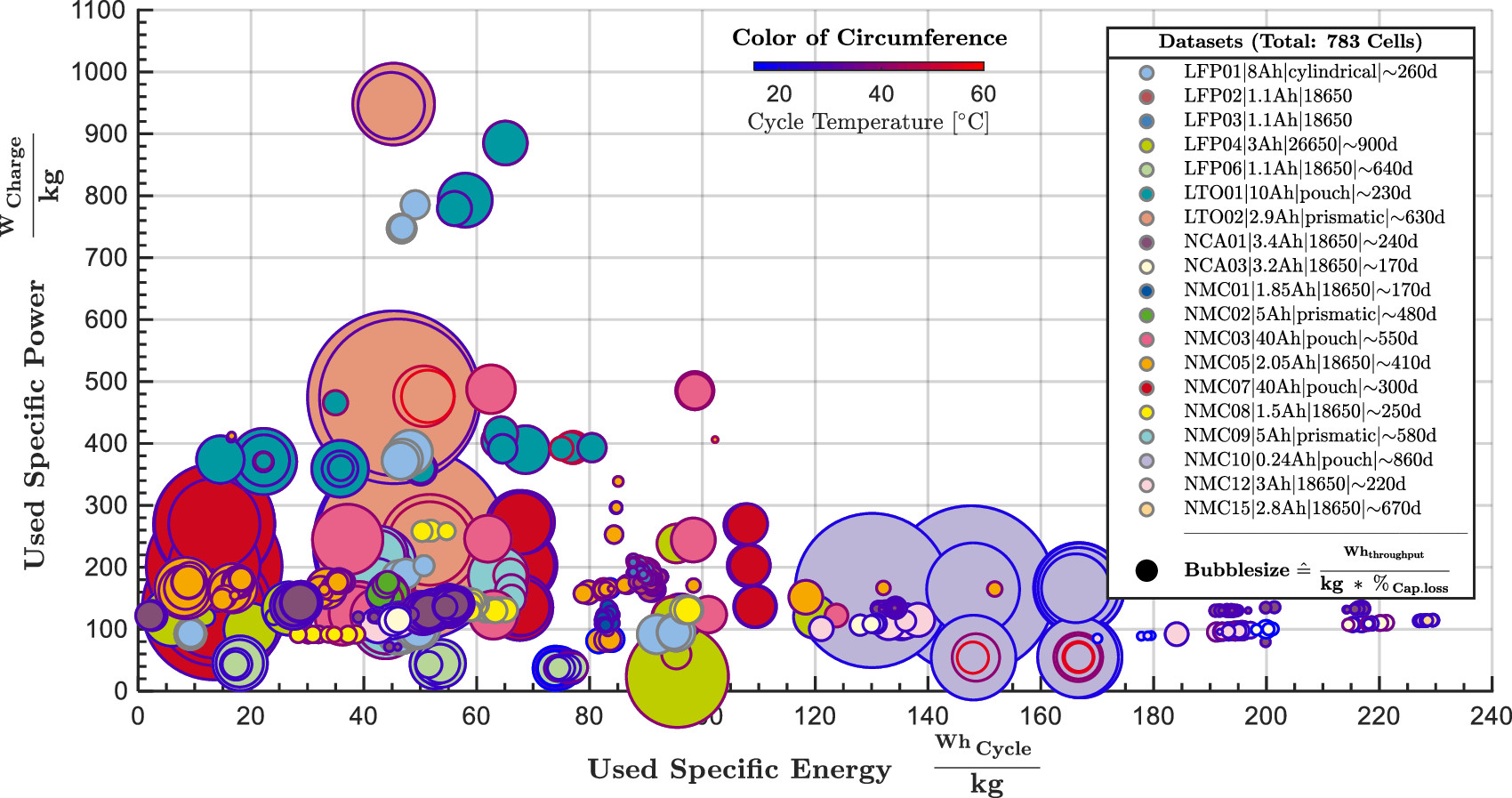
Lower-cost batteries often are longer duration and lower power.
Why Lithium Batteries are Rated For Hour Duration
“We define duration as the length of time a storage system can generate at full output before needing to recharge,” NREL writes.
While a lithium battery could be discharged more slowly, it would not be discharging at its maximum rate. Thus, it’s full power potential is untapped. One thing that stands out from this is that a battery with a high discharge rate has a relatively higher power-to-energy ratio than a longer duration battery. The duration is also a relationship between power and energy. A higher power/energy battery has faster charge and discharge.
One of the practical reasons for showing this specification in this way is economic. One metric for storage cost is in terms of cost/energy such as $/kWh. In economic terms, the faster storage can deliver energy, the faster the cost of storage can be amortized. It is important to optimize cells for the intended use, matching P/E to use.
