Lithium-ion batteries are used not only for small consumer electronics like cell phones and laptops, but also for electric bicycles, electric vehicles and long-term power storage. Though a rare occurrence, lithium-ion batteries can catch fire due to the instability of the liquid electrolytes in the battery structure.
All solid-state lithium metal batteries, which feature improved safety and higher energy density compared with state-of-the-art lithium-ion batteries, are a possible alternative, but they tend to degrade quickly.
A new study led by researchers from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences details why this degradation happens and proposes a simple, effective solution.
The study was published in Science Bulletin on March 13.
“Previously reported laboratory solid-state lithium metal battery prototypes exhibit fast performance decay that challenges their practical application. Revealing the fundamental science towards the problem and gaining an in-depth understanding of the underlying degradation mechanisms of all solid-state lithium metal batteries becomes crucially important for further development,” said Prof. Cui Guanglei, co-corresponding author and the group leader of Solid Energy System Technology Center at QIBEBT.
To understand how these batteries degrade over time, the researchers used an imaging technique called synchrotron X-ray computed tomography (SXCT). SXCT scans produce highly detailed, three-dimensional images of objects without damaging them.
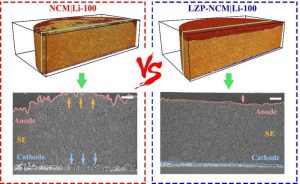
They analyzed the batteries with NCM (LiNi0.8Co0.1Mn0.1O2) cathode active material, LPSC1 (Li6PS5Cl) solid electrolyte and lithium metal anode, known as NCM | Li. Then, they took SXCT scans of the batteries after zero cycles, 50 cycles, and 100 cycles.
By analyzing the images of the cathode and anode after 100 cycles, the researchers uncovered a failure between the two electrodes mediated by uneven fluctuations in lithium-ion flux. Cracked particles of NCM were breaking off the cathode and becoming isolated. The electric current was then focused on the remaining active regions of the cathode. On the parts of the anode facing the active NCM particles on the cathode, there was a higher likelihood of lithium dendrite growth. This could cause many problems for batteries, including reduced capacity and safety.
“For the first time, our work validates that inhomogeneous lithium-ion flux due to heterogeneous electrochemical reactions at the cathode zone dominates the exacerbated interfacial side restrictions and lithium dendrites at the lithium metal anode zone,” said Dr. Zheng Yue, first author of the study.
“This can be effectively resolved by using a surface coating on the cathode material,” said co-corresponding author Dr. Ma Jun. The researchers covered the cathode with a coating made of LiZr2(PO4)3 (LZP) and tested it again, doing additional scans of the battery with the surface coating after 100 cycles. The batteries with the coated cathode had better capacity retention and decreased capacity decay.
“We hope this will open new avenues for research into the current limitations of solid-state batteries,” said co-corresponding author Prof. Sun Fu. “Further development will focus upon the quantification of local current density in solid state batteries, the suppression strategy of anode lithium dendrite, strengthening of the internal contact of the cathode, and improving the critical current density.”
