
Currently, most cathode materials used in batteries for electric vehicles are layered oxides composed of nickel for over 60% of the transition metals. Using nickel-rich layered oxide is advantageous in establishing the mileage of an electric vehicle due to its high energy density, but its usage is limited by instability in the supply and demand of nickel raw materials.
As an alternative, researchers have focused on spinel cathode materials that use manganese as the main element, considering manganese is traded at a price of about 1/17 of nickel in the international spot market; however, the rapid decline in lifespan was an obstacle to commercialization.
The Korea Institute of Science and Technology announced that Dr. Jihyun Hong’s research team at the Energy Materials Research Center identified the cause of the rapid decline in lifespan, a chronic problem of high-capacity manganese-based spinel cathode materials. This team worked on significantly increasing the possibility of commercializing lithium batteries with manganese cathode materials as next-generation electric vehicle batteries. Their results are published in the journal Advanced Energy Materials, and the paper was featured on its cover.
Manganese-based spinel cathode materials can theoretically store energy with a high density comparable to nickel-based commercial cathode materials. Considering the price of metal raw materials, the energy density per price for manganese-based spinel cathode could reach 2.8 times that of nickel-based cathodes. However, when using the battery at full capacity, a rapid decrease in lifespan occurred; as a result, only approximately 75% of the theoretical value could be stored.
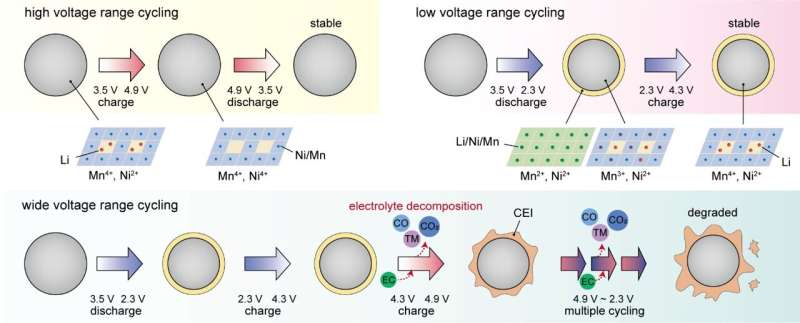
It has been established that the trivalent manganese (Mn3+) that forms during the charging and discharging process of manganese-based spinel cathode materials distorts the crystal structure of the material, leading to the elution of manganese into the electrolyte and eventually causing a reduction in the lifespan of the cathode material. As a result, most research has focused on suppressing the formation of trivalent manganese.
Contrary to mainstream academic theories, Dr. Hong’s team at KIST recently discovered that cathode materials exhibit excellent lifespan characteristics, even when trivalent manganese is formed, if the operating voltage range of the battery is adjusted. The research team used advanced material characterization techniques, including synchrotron radiation techniques, to interpret the phenomena that existing theories cannot explain. Through the analyses, the researchers identified that the side reaction at the interface between the cathode material and electrolyte during the repeated charging and discharging process is the cause of lifespan reduction.
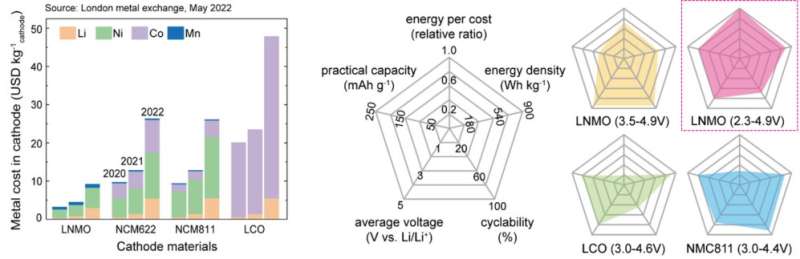
The research team further presented a key strategy to dramatically improve the lifespan of manganese-based materials by stabilizing the cathode-electrolyte interface. As an example of this strategy, introducing an EC-free electrolyte resulted in a 62% improvement in lifespan compared to commercial electrolytes. This improvement results in the highest capacity retention and rate capability among the performances of manganese-based spinel cathode materials simultaneously using nickel and manganese redox reactions reported so far.
Dr. Hong of KIST said, “Through this research, KIST presented a new methodology for commercializing manganese-based high-energy cathode materials, which will be a catalyst for the expansion of electric vehicles. If academia and industry focus on applying the interface stabilization technology of nickel-based cathode materials, which has accumulated a lot of capabilities, to manganese-based next-generation cathode materials, we expect that Korean companies in the automobile industry could maintain a higher level of competitiveness in the future.”





