
Another big grid-scale storage battery will be installed in South Australia’s mid-north in the same region as the Tesla and Neoen big battery at Jamestown.
Tilt Renewables will connect a solar and battery system to its existing wind farm near Snowtown.
It also plans to put a pumped-hydro storage project in a disused quarry at Highbury in Adelaide’s north-east.
What are the plans?
The pumped hydro venture — 300 MW/1350 MWh — would be able to store enough power to exceed the output of the South Australian Government’s temporary diesel-fired Adelaide power plant for up to 4.5 hours.
At Snowtown, a 44 MW solar farm and 21 MW/26 MWh battery would be financially supported by South Australian taxpayers, through a $7.1 million grant from the state renewable technology fund.
How will it compare to the Tesla battery?
The proposed battery will be about one-fifth as powerful and will have one-fifth of the storage of the Tesla and Neoen one, which is already feeding into the electricity grid.
There is also a 30 MW battery due to start operating within months aimed at improving security of power supply on Yorke Peninsula.
In the state’s Riverland, the Lyon Group is involved in another solar farm and battery storage venture.
Will it reduce electricity bills?
Development approvals are still needed for the Tilt ventures, but South Australian Energy Minister Tom Koutsantonis said they would help drive down power prices.
“More renewable energy means cheaper power for South Australians,” he said.
“This planned new solar and battery farm in the mid-north and pumped hydro power plant in Highbury will add a huge amount of additional competition to our system.”
Tilt Renewables chief executive Deion Campbell said the additional infrastructure at Snowtown would create the biggest co-located wind, solar and battery facility in Australia.
He said the evening peak in wind force and daytime peak for solar would combine to help meet electricity demand and both Tilt proposals would lead to less power price volatility.
“Storage has always been a key component of an electricity system and pumped hydro allows renewable electricity to be stored and used when required, without introducing carbon into the equation,” he said.
Fallout from the Tesla virtual power plant idea continues
Meanwhile, an Australian energy company has hit out at the South Australian government over its plan to launch the world’s largest virtual power plant that will see at least 50,000 homes in South Australian given solar panels and batteries.
ShineHub — a Sydney based company — claims it was the first to come up with a similar idea and that it launched an almost identical concept last year in South Australia.
“I think it’s great that South Australia is moving in this direction, it’s definitely what the market needs,” co-founder of ShineHub Jin Kim told the ABC.
“However it was a little bit disappointing to see that the South Australian government did not make this roll-out available — or as an open tendering process for many of the Australian companies, not just ourselves — that could also deliver the exact same service or program.
“We really do hope that the South Australia government will consider a lot more Australian companies for the future plans because there are so many fantastic companies out there.”
Elon Musk is ‘Iron Man’
Mr Kim acknowledged the mastermind behind Tesla, Elon Musk, was leading the way in renewable energy.
But he hoped other smaller, local companies would get the opportunity to work on large-scale project in the years ahead.
“Elon Musk, when we talk about superheros, he is Iron Man, you know he has done such great things for the market itself,” he said.
“They are a fantastic but they are also an international company, for me I am not the Iron Man of renewable energy in Australia… but we really had a focus of how do we get an Australian company to achieve greater heights and help the Australian communities at the same time.”
Government defends approach to project
The Energy Minister, Tom Koutsantonis, argues that all businesses had an opportunity to pitch projects to the government.
“We launched a $150m Renewable Technology Fund and invited all renewables companies to pitch projects into that would benefit SA customers, grow our renewables industry and speed up tech development,” he said.
“That was a competitive, independent process and this project was one of the successful applicants, along with many others, including SA companies.”
Mr Koutsantonis said Tesla was not manufacturing the solar panels, but supplying the batteries for the project.
“In fact, the contract with Tesla includes a requirement that where possible the solar panels to be manufactured locally. This project alone will create 500 direct and indirect jobs in SA,” he said.


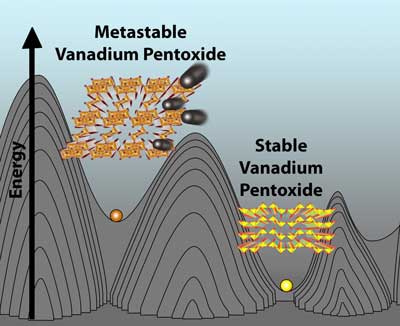 A multi-institution team of scientists led by Texas A&M University chemist Sarbajit Banerjee has discovered an exceptional metal-oxide magnesium battery cathode material, moving researchers one step closer to delivering batteries that promise higher density of energy storage on top of transformative advances in safety, cost and performance in comparison to their ubiquitous lithium-ion (Li-ion) counterparts.
A multi-institution team of scientists led by Texas A&M University chemist Sarbajit Banerjee has discovered an exceptional metal-oxide magnesium battery cathode material, moving researchers one step closer to delivering batteries that promise higher density of energy storage on top of transformative advances in safety, cost and performance in comparison to their ubiquitous lithium-ion (Li-ion) counterparts. The new age energy storage bandwagon has quite possibly hit the buffers, courtesy of a legislative amendment in the polymetallic-rich Democratic Republic of Congo (DRC).
The new age energy storage bandwagon has quite possibly hit the buffers, courtesy of a legislative amendment in the polymetallic-rich Democratic Republic of Congo (DRC).
