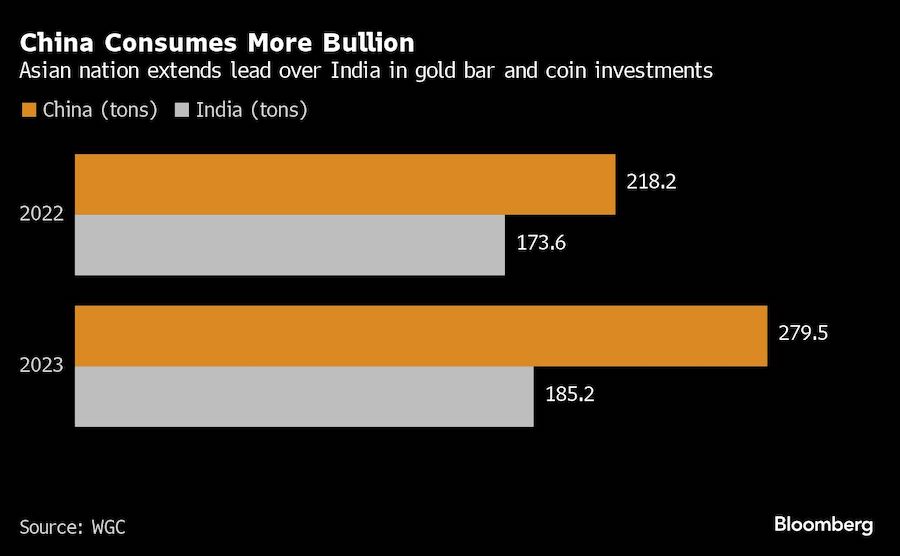
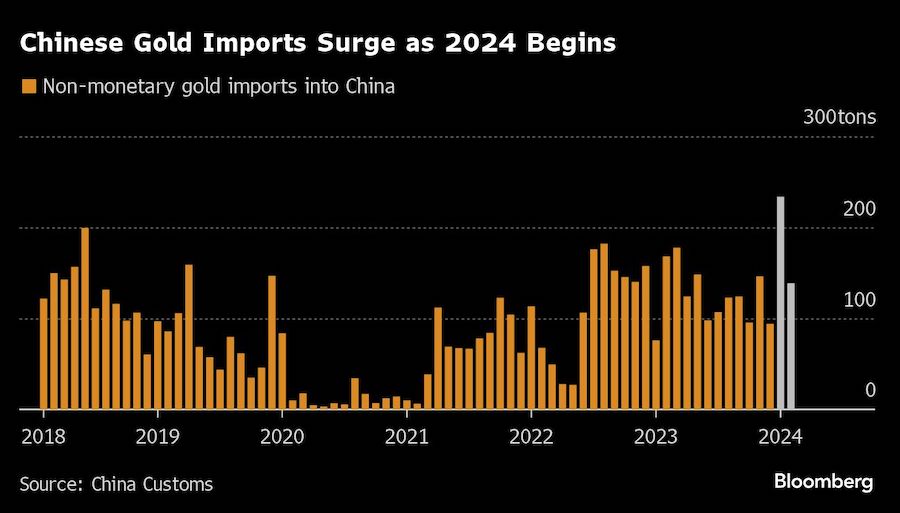
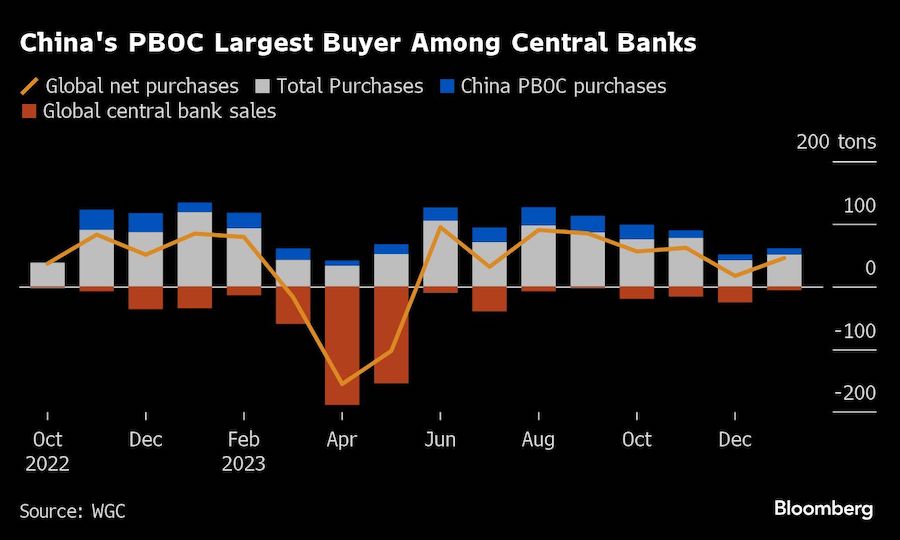
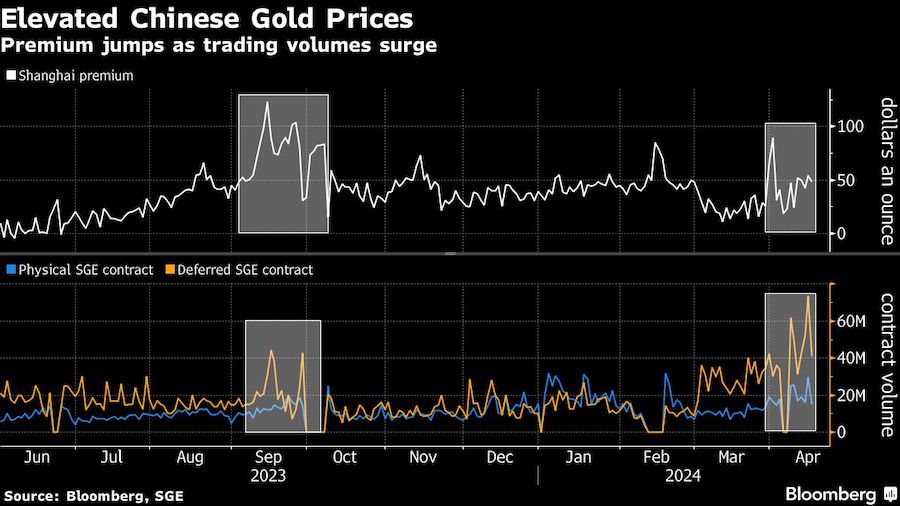
BHP Group Ltd. has approached Anglo American Plc about buying the 107-year-old company in a move that offers to spark the biggest shakeup of the global mining industry in over a decade.
Anglo American said late Wednesday that it received an unsolicited all-share merger proposal from the world’s biggest miner, after Bloomberg reported that BHP was considering a potential takeover offer. Anglo’s board is reviewing the proposal and it cautioned that there is no certainty an offer will be made. Anglo said BHP’s proposal was conditional on the company first splitting off its South African platinum and iron ore units.
If successful, a takeover would mark a return to large-scale dealmaking for BHP, which has revived its appetite for transformational acquisitions in the past couple of years under chief executive officer Mike Henry.
Anglo American has long been viewed as a potential target among the largest miners, particularly because it owns big South American copper operations at a time when most of the industry is seeking to expand in copper. However, suitors have been put off by its complicated structure and mix of commodities, and especially its deep exposure to South Africa.
Anglo has faced a series of major setbacks over the past year, as prices for some of its key products plunged, while operational difficulties have forced the company to slash its production targets — driving down its valuation and leaving the company vulnerable to potential bidders.
Anglo’s shares have dropped 12% over the last 12 months, giving the company a market value of £27 billion ($34 billion). BHP, which trades in London and Sydney, has a market value of about $149 billion.
A successful takeover would represent the first mega deal among the world’s biggest diversified miners in over a decade. BHP and its biggest rivals spent years on the sidelines after a series of disastrous transactions, but have warmed up to the prospect of dealmaking after reassuring investors that they have learned from past mistakes.
BHP last year bought copper producer OZ Minerals Ltd. for about $6.4 billion in its first major purchase in years, but has otherwise focused until now on selling assets such as oil, gas and coal.
For BHP, the clear lure for any deal would be Anglo’s copper assets. The world’s biggest miners are all seeking to expand their copper production in anticipation of rising prices as demand for electrification rises while future supplies look constrained.
Anglo’s South American copper business has long been sought after by bigger players in the industry, although it has recently faced setbacks and had to reduce its copper production forecasts.
It’s also possible that the now-public proposal for Anglo could draw out other potential bidders. No. 2 miner Rio Tinto Group has also been investing in copper production, while Glencore Plc last year made an unsuccessful offer for Teck Resources Ltd., which has a coveted copper business, before eventually reaching a deal for the Canadian company’s coal assets.
While Anglo’s valuation may make it more attractive, it remains a highly complicated business. The company owns majority stakes in two South African-listed miners — Anglo American Platinum Ltd. and Kumba Iron ore Ltd. — and is the majority owner of diamond miner De Beers. It also has a long and complicated relationship with South Africa, where the state pension fund manager is its biggest shareholder.
BHP’s proposal was to first hand Anglo’s stakes in the two South African businesses to the smaller company’s investors before proceeding with a takeover, Anglo said. The two parts of the proposal would be “interconditional,” it said.
Anglo’s other operations include copper, nickel, steelmaking coal and Brazilian iron ore, as well as the iconic De Beers business.
Both companies are also investing in new fertilizer businesses — BHP is building a massive potash mine in Canada, while Anglo is developing a polyhalite mine on the east coast of England.